









Introduction
Case Studies

Infection Control
No foaming cleaning
From three different perspectives: compatibility of cleaning and disinfection equipment, rinsing water consumption, and cleaning efficiency, JUSHA Multi-Enzyme Cleaner adopts foam-free formula technology to ensure cleaning power while being compatible with mainstream brand cleaning and disinfection devices and reducing rinsing water consumption to improve cleaning efficiency.
Efficiently remove biofilm
Using the world's leading genetically engineered biological enzyme technology and compounded with high-performance surfactants, the multi-enzyme cleaning agent has a biofilm removal rate of 99.99%, achieving time-saving, labor-saving and efficient decontamination.
Lead-free
The entire range of sterilization monitoring chemical indicators uses high-precision lead-free color-changing ink technology to avoid the possibility of lead contamination of medical staff and surgical instruments from the source.
Safety
From the product design, production and inspection end, we can realize the transparent visualization of class 5 chemical integrator tablets in manufacturers and medical institutions, prevent customers from using bad integrator with missing tablets, try our best to improve the convenience and safety of customers,
and solve the problem of Clinical problems such as delayed surgery due to missing tablets.
Why Infection Control Solutions?
Normally medical institutions usually need to purchase infection control consumables from multiple suppliers, and decentralized supply chain management may lead to unstable supply timeliness, cumbersome supply terminal management, inconsistent quality standards, high procurement costs, low efficiency and other issues. The overall infection control consumables solution can reduce coordination difficulties between multiple suppliers, poor compatibility during the trial process of different brands of products, etc., and uniformly conduct quality management and monitoring in accordance with medical device standards, through customized supply and inventory management , integrated compatibility formula design helps medical institutions achieve cost control and optimization, improve procurement efficiency and reduce procurement costs.
What is infection control solution?
JUSHA infection control solution is centered on the safe and efficient reprocessing of reusable surgical instruments; it is centered on ensuring the health of patients and medical staff. Oriented to effectively prevent cross-infection, this solution integrates advanced bubble-free biofilm cleaning technology and lead-free color-changing ink formula to ensure cleanliness, sterility and safety during instrument reprocessing, and improve the work efficiency of the department. .
JUSHA provides three series of products covering the reprocessing of medical devices: medical cleaning agents; non-woven sterilization packaging and sterilization chemical indicators.
We are committed to promoting the development of medical cleaning agents towards low foaming, high decontamination and high biofilm removal rates, and sterilization chemical indicators towards lead-free, making your work more accurate, safe and efficient.
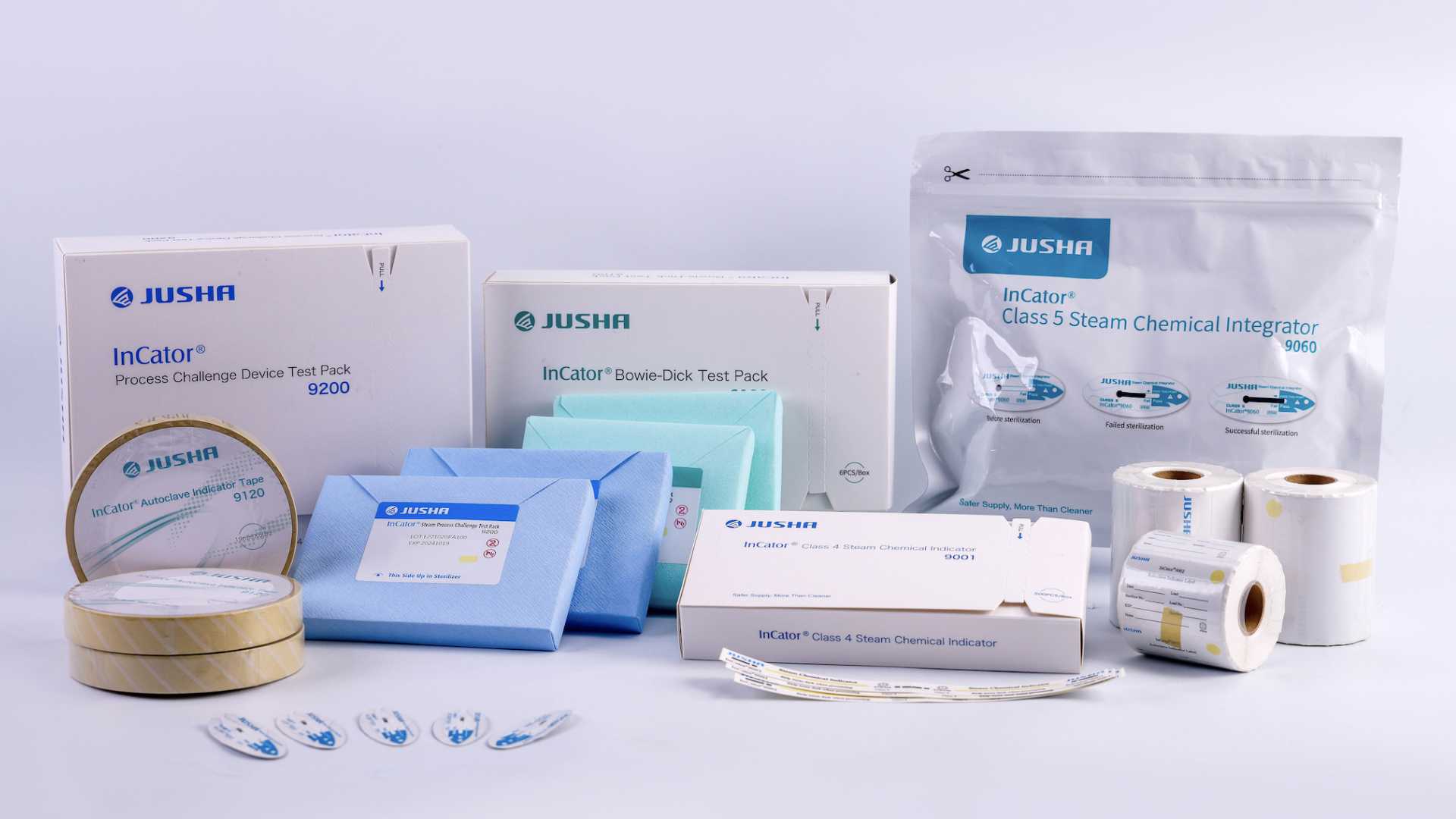
This solution is based on multi-enzyme cleanser, instrument lubricants, alkaline cleanser, non-woven wrap, autoclave indication tape, Bowie Dick test pack, class 5 integrator and other products. Places such as sterilization supply centers, endoscopy centers, third-party regional sterilization supply centers, dental clinics, pharmaceutical factories and biological laboratories, etc.

Product Features
Medical cleaning agent
Clean surface dirt, moisturize instruments, lubricate joints and remove rust stains on contaminated reused surgical instruments, including endoscope multi-enzyme cleaner, full-effect multi-enzyme cleaner, powerful multi-enzyme cleaner, and super-concentrated multi-enzyme cleanser, alkaline cleanser, special lubricants for surgical instruments, instrument rust removers, moisturizing gels, ready-to-use foam multi-enzyme cleaning agents, etc.
Non-woven sterilization packaging:
The cleaned surgical instruments are packaged to form a 180-day sterile barrier system together with the instruments after sterilization, including SMS type 45g/m2, 60g/m2 non-woven wrap, etc.
Sterilization chemical indicators:
Monitor key parameters such as temperature, time, and steam quality during the instrument sterilization process, including steam class 5 chemical integrator, indicator label, autoclave indicator tape, Bowie Dick test pack with early warning, Bowie Dick standard test pack and process challenge device test pack with a class 5 integrator inside, class 4 indicator, etc.
Case Studies